What’s on a Food Label?
The Nutrition Education and Labeling Act of 1990 set the requirements for certain label information to ensure that food labels truthfully inform consumers about the nutrients and ingredients in the package. In 2012, FDA reviewed the details of this information, but in general, every packaged food must state the following:
- The common or usual name of the product.
- The name and address of the manufacturer, packer, or distributor.
- The net contents in terms of weight, measure, or count.
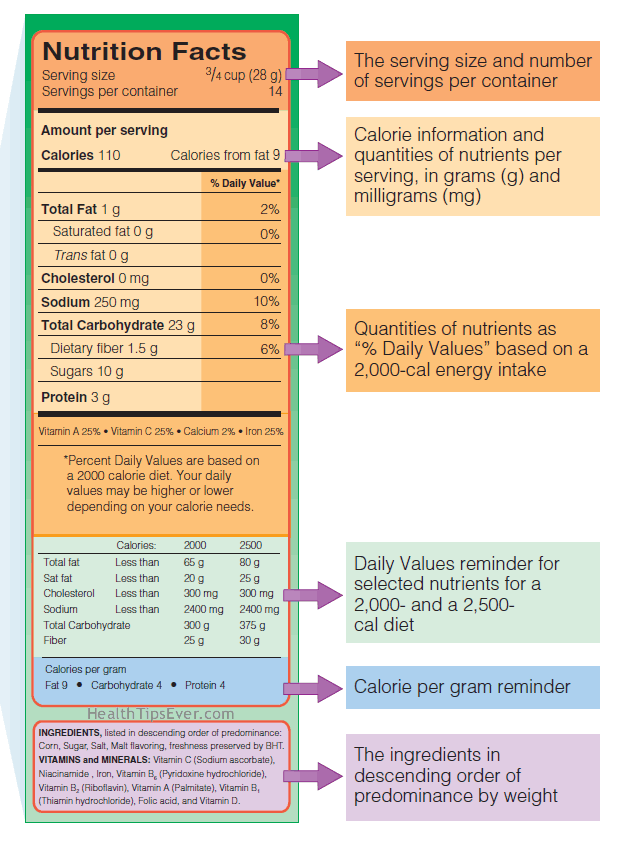
- The nutrient contents of the product (Nutrition Facts panel).
- The ingredients in descending order of predominance by weight and in ordinary language.
- Essential warnings, such as alerts about ingredients that often cause allergic reactions or other problems.
Not every package need display information about every vitamin and mineral. A large package, such as a box of cereal, must provide all of the information just listed. A smaller label, such as the label on a can of tuna, provides some of the information in abbreviated form. A label on a roll of candy rings provides only a phone number, which is allowed for the tiniest labels.
This cereal label maps out the locations of information needed to make wise purchases. The text provides details about each label section. Labels may also warn consumers of potential allergy risks.



I was shocked when I found out what is causing all these food allergies. A friend’s daughter has a serious fish allergy. If she smells fish cooking, it means a hospital trip. The epi pen will help get her there maybe. You can read the regulations for yourself. Pharmaceutical companies can “self-affirm” GRAS (generally recognized as safe) ingredients. Basically, they decide something like refined GMO soy oil should be GRAS. They do one study, have some experts review it, then they are free to use it without submitting anything to the government and it becomes a protected trade secret. How can the FDA protect the public when even they don’t know what is in pharmaceutical products?